Friday, 25 December 2015
Tuesday, 3 November 2015
Ernest Beutler (1928 - 2008)
Beutler was one of the key clinician scientists involved in the development of Cladribine. Read his oral history here, and watch his acceptance speech of the Wallace H. Coulter Award for Lifetime Achievement in Hematology here.

Ernest was also the father of Bruce A Beutler (*1957), who happened to become the 2011 Nobel Prize Laureate, together with Jules A Hoffmann, for "their discoveries concerning the activation of innate immunity".

Here's an interview with Bruce, revealing among other things, that he started working in Dad's lab from the age of 14...

Ernest was also the father of Bruce A Beutler (*1957), who happened to become the 2011 Nobel Prize Laureate, together with Jules A Hoffmann, for "their discoveries concerning the activation of innate immunity".

Here's an interview with Bruce, revealing among other things, that he started working in Dad's lab from the age of 14...
Friday, 30 October 2015
Thursday, 29 October 2015
Re-purpose Cladribine !
Here's the email I sent Chuka Umunna on 18 Oct:
Dear Mr Umunna
I
am contacting you about the drug re-purposing Bill to ask for your
support to see it become Law. I believe it comes before the house on
November 6. I attach some documents setting out the case for the Bill
and please excuse me if you are already familiar with the arguments.
Breakthroughs
in research have meant that several existing drugs have been found to
be effective in treating conditions other than the ones they were
originally made and patented for. They are referred to as “off patent”
drugs. Repurposing these could have potentially huge benefits for many
people suffering from conditions like multiple sclerosis (MS) and Cancer.
They can be supplied at low cost by Generic Drug companies. The annual
treatment for a patient may be less than £1,000 versus over 20x the cost
for patented alternatives if in fact they exist. The savings would be
of great benefit to patients, the NHS and the taxpayer, and could lead
to treatment for many people whose treatment is denied by NICE on the
grounds of cost.
However,
drugs need to be licensed and approved for their new uses in order to
be made available and there is little commercial incentive for
pharmaceutical companies, who normally sponsor this process, to do so
for off patent treatments. This is largely because prices generally fall
a great deal once the patent has expired. There
is no mechanism in place to enable licensing of drugs for uses other
than their original purpose. Even if a doctor can refer to strong
evidence in support of prescribing an off-patent drug to a patient, they
would likely be put off by the potential personal liability they may
face in doing so.
There
are thus political, legal and commercial barriers in place to
prescribing off patent drugs. The Bill seeks to overcome these barriers
by obliging the Government to act
in the public interest through the process of the Medicines and
Healthcare Regulatory Agency (MHRA) to license and approve off-patent,
repurposed drugs for use on the NHS.
The
Bill is strongly supported by medical charities and specialists in the
NHS, including myself - I am a Reader in Clinical Neurology at Queen Mary University of London &
Consultant Neurologist at Barts Health NHS Trust. As a clinical
academic with an interest in MS, I know that early
effective treatment of people with MS is key for a beneficial long term
outcome. I therefore have a particular interest in the re-purposing of
Cladribine, a drug licensed in the UK for patients with hairy cell
leukaemia. There is evidence from phase III trials that Cladribine is
highly effective and safe for people with MS. The annual cost of
Cladribine treatment in MS would be under £1,000 compared to licensed
drugs of lesser efficacy that cost 20x or more. I would be very happy
to expand on this example further if you are interested and have the
time; just call me on my mobile xxx.
For now, I do hope you are able to support the Bill. Please let me know if there is anything further I can do to promote it.
With best wishes
Sincerely Yours,
Klaus Schmierer
I'll keep watching out for comments by my MP, over and above his auto-reply...
Thursday, 22 October 2015
Cladribine
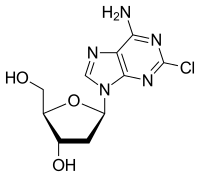
Cladribine (trade names Leustatin, Litak and Movectro) is a drug used to treat hairy cell leukemia (HCL, leukemic reticuloendotheliosis) and multiple sclerosis. Its chemical name is 2-chlorodeoxyadenosine (2CDA).
5-(6-Amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
As a purine analog, it is a synthetic anti-cancer agent that also suppresses the immune system. Chemically, it mimics the nucleosideadenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA. It is easily destroyed by normal cells except for blood cells, with the result that it produces relatively few side effects and results in very little non-target cell loss.
Cladribine was designed by Dennis A. Carson as an anti-lymphocyte compound, while he was at The Scripps Research Institute in La Jolla, California. It was first synthesized at Brigham Young University.The pharmacology and clinical applications were researched by scientists at Johnson and Johnson, which filed the New Drug Application and launched the drug in 1993.
Cladribine was designed based on information about an immune deficiency disease called adenosine deaminase deficiency. Carson described it as "a targeted agent directed against lymphocytes at a time when there was no such thing as targeted agents".
In 2008, Ernest Beutler of The Scripps Research Institute won the Wallace H. Coulter Award for Lifetime Achievement in Haematology from the Coulter Foundation and theAmerican Society of Hematology in part because of the clinical trials he ran, which established cladribine as the most effective treatment for hairy cell leukemia (HCL).
5-(6-Amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
As a purine analog, it is a synthetic anti-cancer agent that also suppresses the immune system. Chemically, it mimics the nucleosideadenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA. It is easily destroyed by normal cells except for blood cells, with the result that it produces relatively few side effects and results in very little non-target cell loss.
Cladribine was designed by Dennis A. Carson as an anti-lymphocyte compound, while he was at The Scripps Research Institute in La Jolla, California. It was first synthesized at Brigham Young University.The pharmacology and clinical applications were researched by scientists at Johnson and Johnson, which filed the New Drug Application and launched the drug in 1993.
Cladribine was designed based on information about an immune deficiency disease called adenosine deaminase deficiency. Carson described it as "a targeted agent directed against lymphocytes at a time when there was no such thing as targeted agents".
In 2008, Ernest Beutler of The Scripps Research Institute won the Wallace H. Coulter Award for Lifetime Achievement in Haematology from the Coulter Foundation and theAmerican Society of Hematology in part because of the clinical trials he ran, which established cladribine as the most effective treatment for hairy cell leukemia (HCL).
Thursday, 1 October 2015
Unrelated Blogger Comments
Contact
NHS
- Address: Dr Klaus Schmierer, Neuroscience Clinical Academic Group, Barts Health NHS Trust, The Royal London Hospital, Whitechapel, London E1 1BB, klaus.schmierer@bartshealth.nhs.uk
- Patient pathway coordinator: Patricia Willams-Falokun, Neuroscience Clinical Academic Group, Barts Health NHS Trust, The Royal London Hospital, Whitechapel, London E1 1BB, patricia.williams-falokun@bartshealth.nhs.uk
- Neurology Infusion and Planned Investigation Unit, The Royal London Hospital, Ward 11D, 11th Floor, Central Tower, Whitechapel, London, E1 1BB, Switchboard: 020 3594 0000, Direct Dial Ward Number: 0203 5940637/0638, Ward Manager: Maria Espasandin maria.espasandin@bartshealth.nhs.uk
- freya.edwards@bartshealth.nhs.uk
- The Royal London Hospital Map: click here for map
ACADEMIC
- Address: Dr Klaus Schmierer, Blizard Institute, Queen Mary University of London, 2 Newark Street, Whitechapel, London E1 2AT. k.schmierer@qmul.ac.uk
- Laboratory Manager: David Holden,for laboratory related issues and queries in relation to grant budgets: Tel: +44 20 7882 2327, Email: d.w.holden@qmul.ac.uk
- Neuroscience & Trauma Centre Administrator: Jyoti Salhan, for issues related to Blizard Institute and the Neuroscience & Trauma Centre, Tel. +44 20 7882 8605, Fax. +44 20 77882 2180 Email: j.salhan@qmul.ac.uk
- Blizard Institute Map: click here for map
Subscribe to:
Posts (Atom)